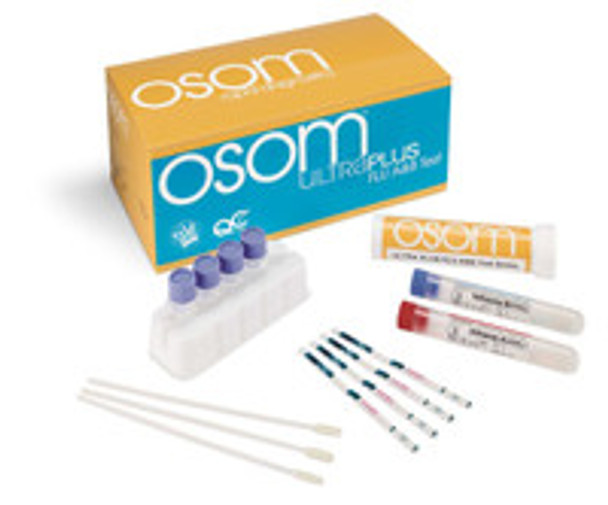
OSOM® Ultra Plus
Respiratory Test Kit OSOM® Ultra Plus Influenza A + B 25 Tests CLIA Waived Sample Dependent 1032 Case of 18
- SKU:
- 1173369_CS
- MPN:
- 1032
- Condition:
- New
- Availability:
- AVAILABLE IN STOCK; READY TO SHIP IN 1-3 DAYS.
Description
Description
The OSOM Ultra Plus Flu A&B Test is an in vitro rapid diagnostic immunochromatography assay intended for the qualitative detection of influenza type A and B nucleoprotein antigens directly from nasal and nasopharyngeal swab specimens from patients with signs and symptoms of respiratory infectionSimple CLIA-waived procedure with pre-measured extraction buffer for swab samplesAccurate, differentiated interpretation of results in 10 minutes to test and treat patients in one office visitMeets the Influenza reclassification requirements for rapid antigen tests
SKU #
1173369
Manufacturer #
1032
Brand
OSOM® Ultra Plus
Manufacturer
Sekisui Diagnostics
Country of Origin
United States
Application
Respiratory Test Kit
CLIA Classification
CLIA Waived for Swab
CLIA Classified
CLIA Waived Sample Dependent
Contents 1
(25) Test Sticks, (25) Sterile Nasal Swabs, (25) Extraction Buffer Vials, (1) Influenza A+ Control Swab, (1) Influenza B+ Control Swab, IFU, QRG, (1) Workstation
Is_Active_Vendor
Y
Is_DSCSA
N
Is_Discontinued
N
Is_Medical_Device
Y
Lot_Tracking_Flag
N
Number of Tests
25 Tests
On_Allocation
N
Product Dating
McKesson Acceptable Dating: we will ship >= 90 days
Purchase Program Type
Standard Purchase
Reading Type
Visual Read
Sample Type
Nasal Swab / Nasopharyngeal Swab Sample
Specialty
Immunoassay
Supplier_ID
4434221
Test Format
Dipstick Format
Test Kit Type
Rapid
Test Name
Influenza A + B
Test Type
Infectious Disease Immunoassay
Time to Results
10 Minute Results
UNSPSC Code
41116144
View AllClose