OraQuick® HCV
Other Infectious Disease Test Kit OraQuick® HCV Hepatitis C Test 25 Tests CLIA Waived 1001-0181 Kit/1
- SKU:
- 805502_KT
- MPN:
- 1001-0181
- Condition:
- New
- Availability:
- AVAILABLE IN STOCK; READY TO SHIP IN 1-3 DAYS.
Description
Description
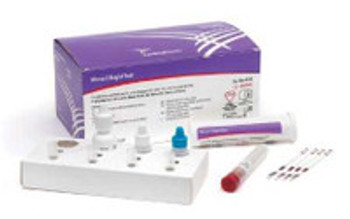
The OraQuick® HCV test is FDA approved for detecting HCV antibodies in fingerstick and venipuncture whole bloodOur simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutesThe OraQuick HCV Rapid Antibody Test is comprised of both a single-use test device and vial containing a pre-measured amount of a buffered developer solutionThe test consists of a sealed pouch with two separate compartments for each componentThe developer solution facilitates the capillary flow of the specimen into the device and onto the assay stripKit includes: 25 divided pouches each containing a test device, absorbent packet, and developer solution vial (each contains 0.75 mL of a buffered saline solution with an antimicrobial agent); 5 reusable test stands; 25 specimen collection loops; package insert
SKU #
805502
Manufacturer #
1001-0181
Brand
OraQuick® HCV
Manufacturer
Orasure Technologies
Country of Origin
United States
Application
Other Infectious Disease Test Kit
CLIA Classification
CLIA Waived
CLIA Classified
CLIA Waived
Contents 1
Single Use Test DevicesBuffered Developer Solution VialsTest StandsSpecimen Collection LoopsPackage Insert
Is_Active_Vendor
Y
Is_DSCSA
N
Is_Discontinued
N
Is_Medical_Device
N
Lot_Tracking_Flag
Y
Number of Tests
25 Tests
On_Allocation
N
Product Dating
McKesson Acceptable Dating: we will ship >= 180 days
Purchase Program Type
Standard Purchase
Reading Type
Visual Read
Sample Type
Whole Blood Sample
Specialty
Immunoassay
Supplier_ID
3883844
Test Format
Test Device Format
Test Kit Type
Rapid
Test Method
Lateral Flow Method
Test Name
Hepatitis C Test
Test Type
Antibody Test
Time to Results
20 Minute Results
UNSPSC Code
41116126
View AllClose