First Response®
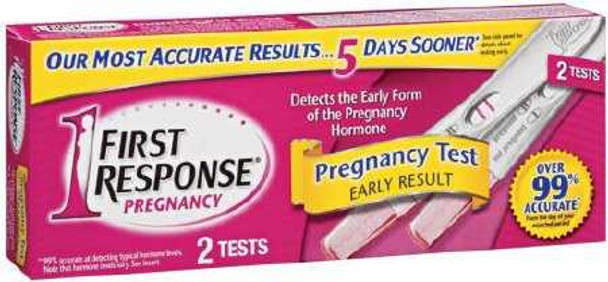
Reproductive Health Test Kit First Response® hCG Pregcy Test 2 Tests CLIA Waived 02260090125 Pack of 1
- SKU:
- 702294_EA
- Condition:
- New
- Availability:
- AVAILABLE IN STOCK; READY TO SHIP IN 1-3 DAYS.
Description
Description
An in-vitro diagnostic home use test device intended for the early detect of pregcyThis test is only intended for individual use at homeIt is not intended for use in a healthcare settingThe test may detect the pregcy hormone (hCG), in some cases, as early as 6 days before the missed period2 Count
SKU #
702294
Manufacturer #
02260090125
Brand
First Response®
Manufacturer
Church and Dwight
Country of Origin
Unknown
Application
Reproductive Health Test Kit
CLIA Classification
CLIA Waived
CLIA Classified
CLIA Waived
Contents 1
(2) Test Devices, Package Insert
FSA Eligible - Buy UOM
Yes
FSA Eligible - Primary UOM
Yes
FSA Eligible - Sell UOM
Yes
Is_Active_Vendor
Y
Is_DSCSA
N
Is_Discontinued
N
Is_Medical_Device
N
Lot_Tracking_Flag
N
NDC Number
02260090125
Number of Tests
2 Tests
On_Allocation
N
Purchase Program Type
Standard Purchase
Reading Type
Visual Read
Sample Type
Urine Sample
Specialty
Chemistry
Supplier_ID
3885243
Test Format
Test Stick Format
Test Kit Type
Rapid
Test Name
hCG Pregcy Test
Test Type
Home Test Device
Time to Results
3 Minute Results
UNSPSC Code
41116205
View AllClose